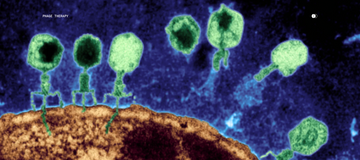
Phage Therapy
for Skin Health
WHITE PAPER
2024
Authored by:
Dr. Shankar Mundluru

Phage Therapy
for Skin Health
01
Introduction
Skin health and the management of skin disorders have been longstanding concerns throughout history. From ancient remedies to modern dermatological advancements, the quest for healthy, radiant skin persists. Among the emerging trends in skincare, phage therapy stands out as a promising avenue for addressing skin-related issues. Here, we delve into the historical evolution of phage therapy, its application in skincare, current solutions provided by companies like Parallel Health, and potential future directions in this burgeoning field.
The history of phage therapy is a fascinating journey that spans over a century, marked by serendipitous discoveries, initial successes, periods of decline, and a recent resurgence of interest. Phage therapy, which involves
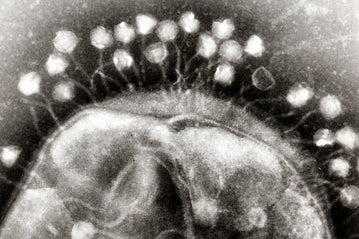
Figure 1. Phages infecting a bacterial cell . Image credit: Graham Beards
02
Historical Evolution of Phage Therapy
using bacteriophages (viruses that target and infect specific bacteria based on their genetic sequences) to treat bacterial infections, has its roots in the pioneering work of scientists like Félix d’Hérelle and George Eliava.
In 1917, Félix d’Hérelle, a French-Canadian microbiologist, made a serendipitous discovery while studying dysentery in World War I. He observed that filtrates from dysentery-infected stools contained viruses that could lyse and kill the dysentery-causing bacteria. D’Hérelle coined the term “bacteriophage” to describe these viruses and proposed their potential use as a therapeutic agent against bacterial
infections.[1,2,3,4]
Following d’Hérelle’s discovery, phage therapy gained traction. In the early 20th century, phage preparations were successfully used to treat various bacterial infections, including dysentery, cholera, and staphylococcal infections[5]. Particularly in Eastern Europe, notably in countries like Georgia and Russia, phage therapy remained a prominent part of medical practice, with dedicated phage therapy institutes established to research and administer phage treatments.

Figure 2. Felix D'Herelle working in his lab, pioneering early phagetherapy. Image credit: Canadian Medical Hall of Fame
However, the advent of antibiotics in the mid-20thcentury led to a decline in the use of phage therapy inWestern medicine. Antibiotics offered broad-spectrumefficacy against multiple strains of bacteria, ease ofproduction, and standardization, overshadowing themore specialized nature of phage therapy. A particularphage is only effective against a single strain of bac-teria, and without the advent of genetic sequencing, theonly way to identify appropriate phages for infectiontreatment was through trial and error. Additionally,technical challenges such as difficulties in phageisolation, purification, stabilization, and standardizationcontributed to the waning interest in phage therapy. [6]
Despite its decline in the West, phage therapycontinued to be practiced in Eastern Europe [7], particularly in Georgia, where the Eliava Institute ofBacteriophages, Microbiology, and Virology wasfounded by George Eliava in 1923. The institute playeda pivotal role in advancing phage therapy research andadministering phage treatments for various bacterialinfections. However, the aforementioned technicalchallenges still hindered its widespread adoptionthroughout the world.[8]
03
Resurgence of Interest inPhage Therapy
The resurgence of interest in phage therapy in recentdecades has been driven by several key factors. Mostimportantly, the global crisis of antibiotic resistance hasunderscored the urgent need for alternative treatmentsto combat drug-resistant bacterial infections. [4]
Phagetherapy offers a promising solution to this challenge, asbacteriophages can target and kill specific antibiotic-resistant bacterial strains without contributing to furtherresistance. [9] More about the power of phages tocombat antibiotic resistance will be discussed laterin this document.
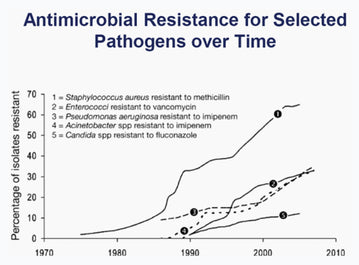
Figure 3. Graph. Image credit: DFWHC Foundation
Furthermore, advances in biotechnology and genomicshave significantly enhanced our understanding of ofbacteriophages and their interactions with bacteria.High-throughput sequencing technologies havefacilitated the identification and characterizationof novel phages with specific lytic activity againstpathogenic bacteria,10 and advances in genetic engineering has allowed us to create phages targetedfor specific bacteria.[11] Additionally, improvements in phage isolation, purification, and formulation have enhanced the efficacy and safety of phage-based therapies, making them more viable options forclinical use. We cannot underscore the difficulty increating treatment formulations with stable phageconcentrations, as phages notoriously disintegrate inalmost all mediums. However, with recent technologicaladvances, Parallel Health has been able to create highconcentration formulations with up to 10B phages perml in formulations that are stable at room temperaturefor at least 8 months. Almost all other phage companieshave only been able to create formulations withexponentially lower concentrations of phages that are not stable for as long.
Moreover, there have been notable success storiesin recent years demonstrating the efficacy of phagetherapy in treating various bacterial infections, includingthose caused by antibiotic-resistant pathogens.[12,13,14]These clinical trials and case studies have generatedexcitement among researchers, clinicians, andpatients, fueling further research and developmentin the field.[15,16,17]
Additionally, regulatory agencies in various countrieshave begun to recognize the potential of phagetherapy as a therapeutic option for antibiotic-resistant infections.[18,19,20] Regulatory support has encouraged investment in phage therapy research and developmentby pharmaceutical companies, biotechnology startups,and academic institutions, leading to accelerated progress in the field, especially due to the extremely safe nature of phages with its relatively low to zero sideeffect profile. [21]
Finally, collaborative efforts among researchers,clinicians, industry partners, and regulatory agencies have been instrumental in advancing phage therapy as a viable treatment option. Global initiatives aimed at facilitating collaboration, sharing resources, and accelerating the translation of phage therapy fromthe laboratory to clinical practice have fostered a supportive ecosystem for research and development in the field. [22]
04
Application of PhageTherapy in Skincare
The skin, our largest organ, is a complex ecosystemteeming with diverse microbial communities thatcontribute to its health and overall well-being. These microorganisms, collectively known as the skinmicrobiome, play a pivotal role in maintaining skinhomeostasis, regulating immune responses, andprotecting against pathogenic invaders. [23,24] However,disturbances in the delicate balance of the skinmicrobiome, known as dysbiosis, can predisposeindividuals to various dermatological conditions,including acne, eczema, and rosacea. [25,26].
Recognizing the importance of maintaining a healthyskin microbiome, researchers and skincare expertshave turned their attention to phage therapy as apromising approach to skincare. Phage therapy offers atargeted and precise method for combating pathogenicbacteria while preserving the symbiotic balance ofthe skin microbiota. The specificity of bacteriophages make them ideal candidates for selectively eliminating harmful bacteria without disrupting the beneficial microflora of the skin. Parallel Health has addressed these obstacles, respectively, through its Skin Discovery Test
and Custom Active Phage Serums, both of which will be expanded upon further later in this document.
The aforementioned phage-based products come in various formulations, including serums, creams, and spot treatments, each designed to target and address different skin issues. By harnessing the natural specificity of bacteriophages, these skincare formulations offer a promising alternative to conventional antibiotics, with the potential to mitigate the risk of antibiotic resistance and minimize adverse effects on the skin microbiome.
Phage-based skincare products work by delivering active bacteriophages directly to the skin’s surface, where they seek out and selectively target bacteria responsible for skin conditions such as acne, eczema, and rosacea[27]. Once bound to their bacterial hosts, phages inject their genetic material, hijacking the bacterial machinery to replicate and propagate, ultimately leading to the destruction of the targeted bacteria. Importantly, phages are highly specific, targeting only the intended bacterial strains while leaving the rest of the skin microbiota and the human cells completely unharmed.[28]
As research in the field of phage therapy continues to advance, we can expect to see further innovations in phage-based skincare products, offering individuals tailored solutions for maintaining healthy and radiant skin. By harnessing the power of bacteriophages, skincare professionals can revolutionize the way we approach skincare, ushering in a new era of precision skincare that prioritizes the health and balance of the skin microbiome.
05
More About the Advantages Over Traditional Antibiotics
Phage therapy represents a paradigm shift in skincare, offering a multitude of advantages over traditional antibiotics that resonate with the evolving landscape of dermatological care. Unlike antibiotics, which wield broad-spectrum antimicrobial activity capable of indiscriminately targeting both pathogenic and beneficial bacteria, phages exhibit unparalleled specificity for their target bacteria. This specificity enables phages to precisely pinpoint and eliminate harmful bacteria while sparing the diverse array of beneficial microorganisms that comprise the skin microbiome. By preserving microbial diversity, phage therapy helps maintain the delicate equilibrium of the skin microbiota, fostering a harmonious environment conducive to optimal skin health and function. [25]
Unlike synthetic antibiotics, which can sometimes elicit adverse reactions or trigger allergic responses in susceptible individuals, phages are inherently biocompatible and unlikely to cause harm to the host. This favorable safety profile, coupled with their targeted mode of action, makes phage therapy an appealing option for individuals with sensitive or reactive skin, offering a gentle yet effective approach to skincare.[30]
Additionally, the localized nature of phage therapy allows for precise and targeted treatment of specific skin concerns, minimizing systemic exposure and reducing the risk of systemic side effects often associated with systemic antibiotic therapy.[34] This targeted approach not only enhances therapeutic efficacy but also minimizes disruption to the delicate balance of the body’s microbiota, promoting overall skin health and well-being.
Moreover, the dynamic nature of phages as self-replicating entities confers a remarkable advantage over traditional antibiotics. Phages possess the innate ability to adapt and evolve in response to changes in bacterial populations, thereby reducing the risk of resistance development—a formidable challenge plaguing antibiotic therapy.[18] This inherent adaptability equips phages with a unique capacity to combat antibiotic-resistant bacteria, offering a beacon of hope in the face of escalating antimicrobial resistance.[29] Furthermore, the issue with antibiotics is that if they don’t completely eliminate a particular bacterial strain, the bacteria mutate and become resistant to that particular antibiotic. On the other hand, since phage therapy is so specific, there is less chance of not completely eradicating a particular strain of bacteria, and if the bacteria does mutate, there is almost always another phage that could target it. Therefore, bacterial resistance towards phages is much less of an issue with phages than that of bacteria.
Furthermore, phages’ natural origins and coexistencewith bacteria in the environment imbue them with a remarkable degree of biocompatibility and safety.
06
Clinical Studies and Efficacyof Phage Therapy
Broad Applications of Phage Therapy
Numerous clinical studies have demonstrated the efficacy of phage therapy in treating bacterial infections, both in vitro and in vivo. One notable example is the successful treatment of chronic bacterial infections, such as chronic otitis, chronic wounds, and chronic urinary tract infections, using phage therapy.[31] For instance, a study published in Frontiers in Microbiology in 2020 evaluated the efficacy of phage therapy in treating chronic otitis caused by antibiotic-resistant Pseudomonas aeruginosa strains. The results showed a significant reduction in bacterial load and clinical improvement in patients treated with phage therapy, highlighting the potential of phage therapy as an alternative treatment for chronic infections.[35]
Phage therapy has also shown promise in the treatment of acute infections, including bacteremia, septicemia, and acute respiratory infections. Clinical trials have demonstrated the rapid and targeted actionof phages against bacterial pathogens, leading toimproved clinical outcomes and reduced mortalityrates compared to conventional antibiotic treatments.For example, a study published in the Journal ofAntimicrobial Chemotherapy in 2019 evaluated theefficacy of phage therapy in treating multidrug-resistant Acinetobacter baumannii infections in criti-cally ill patients. The results demonstrated a significantreduction in bacterial load and mortality rates inpatients treated with phage therapy compared tostandard antibiotic therapy, highlighting the potential ofphage therapy as a life-saving intervention for severebacterial infections.[13,15]
Phage therapy has also shown efficacy in the treatmentof biofilm-associated infections, which are notoriouslydifficult to treat with conventional antibiotics due totheir intrinsic resistance mechanisms and the difficultyof antibiotics to penetrate the biofilm. Clinical studieshave demonstrated the ability of phages to penetrate and disrupt bacterial biofilms, leading to improvedclinical outcomes and reduced rates of recurrence. For example, a study published in the International Journal of Antimicrobial Agents in 2018 evaluated theefficacy of phage therapy in treating chronic biofilm-associated infections in patients with cystic fibrosis. The results showed a significant reduction in bacterialload and improvement in lung function in patients treated with phage therapy, highlighting the potential of phage therapy as a targeted and effective treatment for biofilm-associated infections.[32]
Phage Therapy for Skin
In recent years, there has been growing interest in thepotential applications of phage therapy in skincare,particularly in the management of bacterial skininfections, acne [33] , atopic dermatitis [34] , and otherdermatological conditions. [35,36] Clinical studies haveevaluated the efficacy of phage-based skincareproducts in targeting and eliminating pathogenicbacteria while preserving the symbiotic balance of theskin microbiome.
One study published in the Journal of Drugs in Derm-atology in 2020 evaluated the efficacy of a phage-
based topical gel in treating acne vulgaris. The resultsshowed a significant reduction in acne severity and inflammatory lesions in patients treated with the phage-based gel compared to placebo, indicating the potentialof phage therapy as a novel treatment for acne. [33] Another study published in Dermatology and Therapyin 2019 investigated the efficacy of a phage-based cleanser in reducing bacterial colonization on the skin. The results demonstrated a significant reduction inbacterial load and improvement in skin microbiome diversity in patients using the phage-based cleanser compared to conventional antibacterial cleansers,highlighting the potential of phage therapy as a gentleand effective alternative for skincare. [28]
Furthermore, clinical studies have explored theuse of phage therapy in the treatment of chronicskin conditions, such as atopic dermatitis, eczema,psoriasis, ulcers and chronic wounds. Findingssuggest that phage therapy may help modulate thedysbiotic skin microbiome and reduce inflammation,leading to improved clinical outcomes and quality oflife for patients with chronic skin conditions. By virtueof their specificity and adaptability, phage-basedskincare products offer a personalized approach toskincare that aligns seamlessly with the principlesof precision medicine. By precisely targeting the underlying causes of skin disorders, phage therapy addresses the root of the problem, rather than merely alleviating symptoms—a hallmark of effective and sustainable skincare.[37]
07
Current Solutions
There are several companies who have recentlyentered the skin microbiome and phage therapyspace for skincare solutions. Locus Biosciences and PhagoMed Biopharma, are focusing on the development of personalized phage therapies tailored to individual patients’ skin infections. By leveraging advances in genomics and biotechnology, they aim to identify and characterize bacteriophages with specific
with specific activity against antibiotic-resistant bacteriacommonly associated with skin infections. Additionally,companies like Phage Technologies and Bioharmony Therapeutics are exploring the use of phage therapyin skincare products, including cleansers, serums,and moisturizers. These products are designed todeliver bacteriophages to the skin effectively, targetingbacterial pathogens while promoting skin health andmicrobiome diversity. Furthermore, Phylabiotics has developed a phage based acne serum. However, these solutions are not targeted to individual patients’ specificstrains of bacterial overgrowth, making them not effective for all customers.
Parallel Health, on the other hand, stands as a trailblazer in the realm of phage-based skincare.Unlike other companies which are either focusingon testing or phage therapy, Parallel stands out bycombining both. At the forefront of Parallel Health’s product lineup lies its flagship offering: the Skin Discovery Test—a revolutionary diagnostic tool that empowers consumers to unlock the secrets of their skin’s microbiome. Through a simple set of swabs ofthe face, customers can access a wealth of informationabout their skin’s bacterial composition without theneed for invasive procedures. Leveraging state-of-the-art genetic sequencing technology,
Parallel Health identifies and analyzes the diverse array of bacteria inhabiting the skin, providing valuable insights into the skin’s microbial ecosystem.
Following the Skin Discovery Test, customers receive a comprehensive Skin Health Report meticulously curated based on the results of the test and a detailed health intake form. This personalized report offers aholistic overview of the customer’s skin health, including their skin age, hydration levels, microbial diversityscores, and recommendations for optimal skincare. Armed with this invaluable information, customers areassigned one of eight precision phage serums tailoredto their unique skin type and bacterial profile, ensuringtargeted and effective skincare solutions.In addition to the precision phage serums, Parallel Health offers a range of microbiome-friendly cleansersand moisturizers specially formulated to promoteskin health and microbiome balance.

Figure 4. Parallel's skin health protocol, which includes a SkinDiscovery Test and Active Phage Serum. Image credit: ParallelHealth, Inc.
08
Future Directions
The future of phage therapy in skincare holds immense promise for continued innovation and advancement. A sour understanding of the skin microbiome and its role in skin health deepens, there is growing recognition of the importance of preserving microbial diversity andecological balance.
One potential avenue for future exploration lies in the development of personalized skincare regimens tailored to individual skin microbiome profiles. By leveraging advanced diagnostic tools and genetic sequencing technologies, skincare professionals maybe able to analyze an individual’s skin microbiota and formulate customized skincare solutions optimized fortheir unique needs, perhaps even beyond phage based products. This personalized approach has the potential to revolutionize skincare by maximizing efficacy and minimizing adverse reactions.
These products are meticulously crafted with gentle yet effective ingredients that support the skin’s natural barrier function and promote microbial diversity, fostering a harmonious environment conducive to optimal skin health.Moreover, Parallel Health goes above and beyondby offering personalized compounded skin healthprescriptions designed to target specific skindiseases such as acne, rosacea, and melasma.
These customized prescriptions leverage the powerof personalized medicine to treat dermatologicalconditions, offering individuals tailored solutions fortheir skincare needs.
Through its innovative products and personalizedapproach to skincare, Parallel Health is revolutionizingthe way we approach dermatological care,empowering individuals to take control of their skinhealth and unlock their skin’s full potential. With itsunwavering commitment to excellence and cutting-edge technology, Parallel Health is paving the wayfor a brighter future in skincare—one that prioritizesprecision, efficacy, and personalized care.
Moreover, researchers are increasingly exploring the synergistic effects of phage therapy with other skincare modalities, such as probiotics, prebiotics, and botanical extracts. These combination therapies hold promise for enhancing therapeutic outcomes by leveraging the complementary mechanisms of action of different skincare ingredients. By harnessing the power of phages alongside other beneficial compounds, skincare professionals may be able to address a broader spectrum of skin concerns and achieve superior results.
Another area of focus for future research is the development of advanced delivery systems for phage-based skincare products. Novel delivery systems, such as microencapsulation, liposomes, and nanoparticles, offer opportunities to enhance the stability, bioavailability, and penetration of phages into the skin. By optimizing delivery mechanisms, researchers aim to maximize the therapeutic efficacy of phage therapy while minimizing potential barriers to its effectiveness.
09
Conclusions
Phage therapy represents a paradigm-shiftingapproach to skincare, harnessing the natural specificityand adaptability of bacteriophages to target pathogenicbacteria while preserving the symbiotic balance ofthe skin microbiome. As research and developmentin phage therapy continue to evolve, the potentialfor personalized skincare regimens, synergisticcombination therapies, and advanced delivery systemsis vast.
Companies like Parallel Health are spearheading thistransformative shift in skincare, offering innovativeproducts and solutions that address unmet needsin dermatological care. By collaborating withmultidisciplinary teams of scientists, clinicians,and skincare experts, these companies are drivinginnovation and shaping the future of skincare.
As we embark on this journey towards a new era ofskin health and wellness, it is imperative to prioritizescientific rigor and efficacy in the development anddeployment of phage-based skincare products andtherapies. By leveraging the power of phage therapy,we can unlock new possibilities for personalized, precision skincare that enhances skin health and well-being for individuals worldwide.

Furthermore, ongoing research efforts are focused on elucidating the mechanisms of action of phage therapy in skincare. Researchers aim to gain a deeper understanding of how phages interact with bacterial pathogens on the skin, as well as how they influence the skin’s immune response and overall health. This knowledge will inform the development of more targeted and effective phage-based therapies for a wide range of dermatological conditions, including wound healing, scar management, and anti-aging skincare.
CITATIONS
1. Abedon, S. T. Phage Therapy: Various Perspectives on How to Improve the Art. Methods Mol. Biol. 1734, 113–127 (2018).
2. Sulakvelidze, A., Alavidze, Z. & Morris, J. G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 45, 649–659 (2001).
3. Summers, W. C. Bacteriophage therapy. Annu. Rev. Microbiol. 55, 437–451 (2001).
4. Housby, J. N. & Mann, N. H. Phage therapy. Drug Discov. Today 14, 536–540 (2009).
5. Kutter, E. et al. Phage therapy in clinical practice: treatment of human infections. Curr. Pharm. Biotechnol. 11, 69–86 (2010).
6. Luong, T., Salabarria, A.-C. & Roach, D. R. Phage Therapy in the Resistance Era: Where Do We Stand and Where Are We Going? Clin. Ther. 42, 1659–1680 (2020).
7. Pirnay, J.-P. et al. The phage therapy paradigm: prêt-à-porter or sur-mesure? Pharm. Res. 28, 934–937 (2011).
8. Chanishvili, N., Myelnikov, D. & Blauvelt, T. K. Professor Giorgi Eliava and the Eliava Institute of Bacteriophage. Phage (New Rochelle) 3, 71–80 (2022).
9. Pelfrene, E., Willebrand, E., Cavaleiro Sanches,A., Sebris, Z. & Cavaleri, M. Bacteriophagetherapy: a regulatory perspective. J. Antimicrob.Chemother. 71, 2071–2074 (2016).
10. Drulis-Kawa, Z., Majkowska-Skrobek, G.,Maciejewska, B., Delattre, A.-S. & Lavigne, R.Learning from bacteriophages - advantages andlimitations of phage and phage-encoded proteinapplications. Curr. Protein Pept. Sci. 13, 699–722(2012).
11. Pires, D. P., Cleto, S., Sillankorva, S., Azeredo,J. & Lu, T. K. Genetically Engineered Phages:a Review of Advances over the Last Decade.Microbiol. Mol. Biol. Rev. 80, 523–543 (2016).
12. Schooley, R. T. et al. Development and Use ofPersonalized Bacteriophage-Based TherapeuticCocktails To Treat a Patient with a DisseminatedResistant Acinetobacter baumannii Infection.Antimicrob. Agents Chemother. 61, (2017).
13.Dedrick, R. M. et al. Engineered bacteriophagesfor treatment of a patient with a disseminateddrug-resistant Mycobacterium abscessus. Nat. Med.25, 730–733 (2019).
14. Gordillo Altamirano, F. L. & Barr, J. J. PhageTherapy in the Postantibiotic Era. Clin.Microbiol. Rev. 32, (2019).
15. Chan, B. K., Stanley, G., Modak, M., Koff, J.L. & Turner, P. E. Bacteriophage therapy forinfections in CF. Pediatr. Pulmonol. 56 Suppl 1,S4–S9 (2021).
16.Jault, P. et al. Efficacy and tolerability of acocktail of bacteriophages to treat burn woundsinfected by Pseudomonas aeruginosa (PhagoBurn):a randomised, controlled, double-blind phase 1/2trial. Lancet Infect. Dis. 19, 35–45 (2019).
17.Merabishvili, M. et al. Quality-controlled small-scale production of a well-defined bacteriophagecocktail for use in human clinical trials. PLoSOne 4, e4944 (2009).
18.Kortright, K. E., Chan, B. K., Koff, J. L. & Turner,P. E. Phage Therapy: A Renewed Approach to CombatAntibiotic-Resistant Bacteria. Cell Host Microbe25, 219–232 (2019).
19. Chan, B. K., Abedon, S. T. & Loc-Carrillo, C.Phage cocktails and the future of phage therapy.Future Microbiol. 8, 769–783 (2013).
20. Lin, D. M., Koskella, B. & Lin, H. C. Phagetherapy: An alternative to antibiotics in the ageof multi-drug resistance. World J. Gastrointest.Pharmacol. Ther. 8, 162–173 (2017).
21. Fauconnier, A. Phage Therapy Regulation: FromNight to Dawn. Viruses 11, (2019).
22. Brüssow, H. Hurdles for Phage Therapy (PT) toBecome a Reality. (MDPI, 2019).
23. Meisel, J. S. et al. Commensal microbiotamodulate gene expression in the skin. Microbiome6, 20 (2018).
24. Grice, E. A. & Segre, J. A. The skin microbiome.Nat. Rev. Microbiol. 9, 244–253 (2011).
25. Kong, H. H. Skin microbiome: genomics-basedinsights into the diversity and role of skinmicrobes. Trends Mol. Med. 17, 320–328 (2011).
26. National Academies of Sciences, Engineering,and Medicine, Division on Earth and LifeStudies, Board on Life Sciences, Board onEnvironmental Studies and Toxicology & Committeeon Advancing Understanding of the Implicationsof Environmental-Chemical Interactions with theHuman Microbiome. Environmental Chemicals, theHuman Microbiome, and Health Risk: A ResearchStrategy. (National Academies Press, 2018).
27.Kutateladze, M. & Adamia, R. Bacteriophagesas potential new therapeutics to replace orsupplement antibiotics. Trends Biotechnol.
28,591–595 (2010).28.Natarelli, N., Gahoonia, N. & Sivamani, R. K.Bacteriophages and the Microbiome in Dermatology:The Role of the Phageome and a PotentialTherapeutic Strategy. Int. J. Mol. Sci. 24, 2695(2023).
29.Hasan, M. & Ahn, J. Evolutionary Dynamics betweenPhages and Bacteria as a Possible Approachfor Designing Effective Phage Therapies againstAntibiotic-Resistant Bacteria. Antibiotics(Basel) 11, (2022).
30.Górski, A. et al. Phage Therapy: CombatingInfections with Potential for Evolving fromMerely a Treatment for Complications to TargetingDiseases. Front. Microbiol. 7, 1515 (2016).30.Górski, A. et al. Phage Therapy: CombatingInfections with Potential for Evolving fromMerely a Treatment for Complications to TargetingDiseases. Front. Microbiol. 7, 1515 (2016).
31. Chan, B. K. et al. Phage selection restoresantibiotic sensitivity in MDR Pseudomonasaeruginosa. Sci. Rep. 6, 26717 (2016).
32. Azeredo, J. & Sutherland, I. W. The use of phagesfor the removal of infectious biofilms. Curr.Pharm. Biotechnol. 9, 261–266 (2008).
33. Farfán, J., Gonzalez, J. M. & Vives, M. Theimmunomodulatory potential of phage therapyto treat acne: a review on bacterial lysis andimmunomodulation. PeerJ 10, e13553 (2022).
34. Shimamori, Y. et al. Staphylococcal Phage inCombination with Staphylococcus Epidermidis as aPotential Treatment for Staphylococcus Aureus-Associated Atopic Dermatitis and Suppressor ofPhage-Resistant Mutants. Viruses 13, (2020).
35. Zyman, A., Górski, A. & Międzybrodzki, R. Phagetherapy of wound-associated infections. FoliaMicrobiol. 67, 193–201 (2022).
36.Plumet, L. et al. Bacteriophage Therapyfor Infections: A Review of Animal Models,Treatments, and Clinical Trials. Front. Cell.Infect. Microbiol. 12, 907314 (2022).
37.Albac, S. et al. Efficacy of Bacteriophages in aStaphylococcus aureus Nondiabetic or DiabeticFoot Infection Murine Model. Antimicrob. AgentsChemother. 64, (2020).
38.Cogen, A. L., Nizet, V. & Gallo, R. L. Skinmicrobiota: a source of disease or defence? Br.J. Dermatol. 158, 442–455 (2008).